Isaralgagene civaparvovec (ST-920) for Fabry disease
Last updated Feb. 13, 2025, by Lindsey Shapiro, PhD

What is isaralgagene civaparvovec for Fabry disease?
Isaralgagene civaparvovec (ST-920) is an investigational gene therapy being developed to reduce the abnormal accumulation of certain fatty molecules and slow or halt disease progression in Fabry disease.
Sangamo Therapeutics is developing the therapy, to be given as a one-time intravenous (into-the-vein) infusion.
The gene therapy has received fast track status and was named an orphan drug, and regenerative medicine advanced therapy by the U.S. Food and Drug Administration (FDA). It also won orphan medicinal product designation and priority medicines eligibility in the European Union. Such designations are intended, in various ways, to speed isaralgagene civaparvovec’s clinical development.
While isaralgagene civaparvovec is currently in Phase 1/2 clinical testing, Sangamo announced plans to launch a Phase 3 trial. The FDA advised that one additional study involving 25 Fabry patients may be sufficient to support a regulatory application.
Therapy snapshot
| Treatment name: | isaralgagene civaparvovec (ST-920) |
| Administration: | Being tested in Fabry disease as a one-time intravenous infusion |
| Clinical testing: | Currently being tested in a Phase 1/2 trial; Phase 3 study planned |
How does isaralgagene civaparvovec work?
Fabry disease is caused by mutations in the GLA gene, resulting in a lack of functional alpha-galactosidase A (Gal A), which is an enzyme needed for breaking down certain fatty molecules, such as globotriaosylceramide (Gb3) and lyso-Gb3. When Gal A is deficient, these molecules tend to accumulate to toxic levels and cause damage in various tissues and organs, such as the heart, kidney, nervous system, eyes, and skin.
Isaralgagene civaparvovec is a gene therapy designed to deliver a healthy version of the GLA gene to liver cells. This enables cells to continuously make their own functional version of Gal A, thus facilitating Gb3 breakdown and easing Fabry symptoms.
The therapy is packaged into a modified viral carrier called an adeno-associated virus (AAV) 2/6, which is more specific to the liver. This type of virus is commonly used in gene therapies because it does not cause disease in humans, and it’s relatively easy to manipulate in a laboratory.
According to Sangamo, isaralgagene civaparvovec has a low immunogenicity, meaning it will generally not trigger an immune response that reduces its efficacy, and does not require pretreatment with steroids or other agents to suppress such immune responses. The gene therapy may also eliminate the need for repeat enzyme replacement therapy (ERT) infusions, the current standard of care for people with Fabry.
How will isaralgagene civaparvovec be administered?
In clinical trials, isaralgagene civaparvovec is administered as a single intravenous infusion, at doses ranging from 2.6 trillion vector genomes per kilogram of body weight (vg/kg) to 26.3 trillion vg/kg. The higher dose has been selected for further testing, but it’s currently unknown if that will be the dose used if the therapy is ultimately approved.
Isaralgagene civaparvovec in clinical trials
The safety and tolerability of isaralgagene civaparvovec is being investigated in a global Phase 1/2 clinical trial, called STAAR clinical trial (NCT04046224). This is the first study to test the gene therapy in humans.
The STAAR trial is conducted in two parts. In the dose-escalation part, researchers tested four different doses of isaralgagene civaparvovec — 2.6 trillion vg/kg, 5.3 trillion vg/kg, 15.8 trillion vg/kg, or 26.3 trillion vg/kg — in nine male participants.
Then, in the dose-expansion part, the gene therapy is being tested in five select groups of patients, including those positive for antibodies against Gal A, those without such antibodies, female patients, men and women with heart disease, and men and women with kidney disease.
In this part, isaralgagene civaparvovec is given at the highest dose of 26.3 trillion vg/kg, which was selected based on data from the first patients. As of September 2023, 15 patients had been dosed in the trial’s second part.
Interim data indicate isaralgagene civaparvovec was well tolerated, with the most common side effects being fever, headache, viral infections, fatigue, and cold-like symptoms. No patients showed signs of liver damage that required steroid treatment.
Data have consistently demonstrated an increase in Gal A levels after the infusion, which have been sustained above the normal range for up to three years of follow-up. Consistently, levels of lyso-Gb3 largely decreased in treated patients.
Among the 13 patients who were on ERT at the time isaralgagene civaparvovec was administered, 12 were able to stop using it, with Gal A levels still remaining normal or above normal in all patients for up to 19 months.
For the 13 patients with at least one year of follow-up, results showed stable kidney function. Moreover, scores on the Fabry Outcome Survey adaptation of the Mainz Severity Score Index — a measure of disease severity — significantly decreased by an average of about four points after a year, reflecting a reduction in disease severity. Data likewise indicated significant improvements in life quality and decreases in gastrointestinal symptoms a year after receiving isaralgagene civaparvovec.
Finally, for the seven patients who had antibodies against Gal A associated with ERT use, data showed antibody levels decreased for all of them, and became undetectable in five people (71%), after receiving the gene therapy.
The STAAR trial is set to finish in 2025. Participants who complete the year of follow-up can enter a long-term extension study (NCT05039866), where all will be monitored for up to five more years.
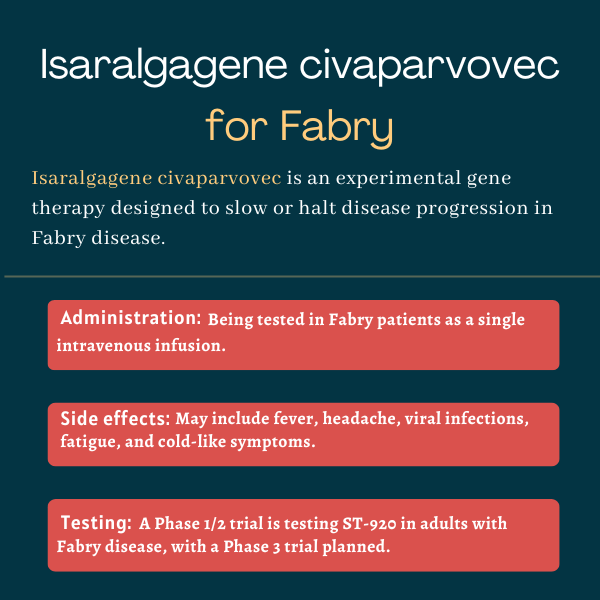
Common side effects of isaralgagene civaparvovec
The most common side effects reported in clinical trials of isaralgagene civaparvovec included:
- fever
- headache
- viral infections
- fatigue
- cold-like symptoms (nasopharyngitis).
Fabry Disease News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Survey: Fabry disease affects family planning among Japanese women
- Fabry disease may raise risk of renal cell carcinoma: Study
- Myeloid bodies may aid in Fabry diagnosis, treatment monitoring
- Life must go on, even when Fabry disease symptoms flare up
- Fabry disease treatment ST-920 improves kidney function: Data
- Unexpected health events are part of my life with Fabry disease
- Blood biomarkers may reflect disease activity, kidney function
- Real-world study shows burden of heart, other complications in Fabry
- Study identifies eye changes in Fabry that could affect vision
- 5 tips for helping teens with Fabry disease to thrive
Related articles