Elfabrio (pegunigalsidase alfa-iwxj) for Fabry disease
Last updated Feb. 22, 2024, by Marisa Wexler, MS

What is Elfabrio for Fabry disease?
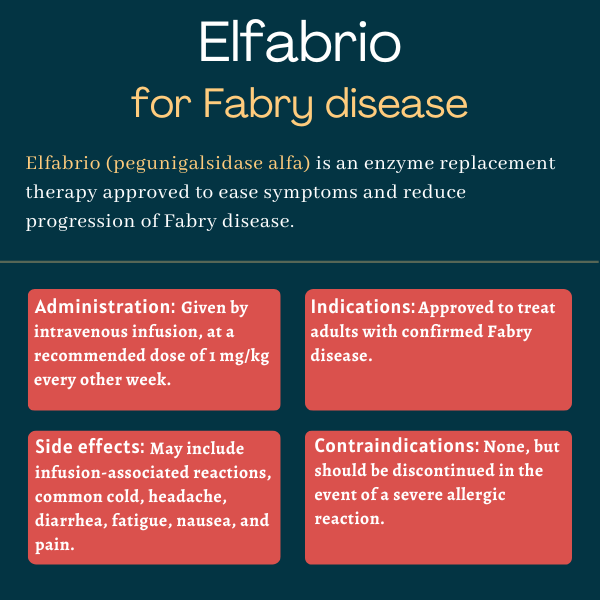
Elfabrio (pegunigalsidase alfa-iwxj) is an enzyme replacement therapy (ERT) approved for adults with Fabry disease. Given via intravenous, or into-the-vein, infusions, the therapy is designed to manage symptoms and slow disease progression.
Formerly known as PRX-102, it was developed by Protalix BioTherapeutics in collaboration with Chiesi Global Rare Diseases. But under a 2018 licensing agreement, Chiesi became solely responsible for developing and marketing the therapy in the U.S.
Therapy snapshot
| Brand name: | Elfabrio |
| Chemical name: | Pegunigalsidase alfa-iwxj |
| Usage: | Enzyme replacement therapy for Fabry disease; can ease symptoms and slow disease progression |
| Administration: | Intravenous infusion |
How does Elfabrio work?
Fabry disease is caused by mutations in the GLA gene, which provides instructions for making alpha-galactosidase A (Gal A), an enzyme needed to break down certain fatty molecules. Without a functional version of the Gal A enzyme, these fatty molecules build up to toxic levels in cells, especially in small blood vessels, and those of the heart and kidneys, causing tissue damage and leading to Fabry symptoms.
As an enzyme replacement therapy, Elfabrio is designed to replace the missing enzyme and reduce the accumulation of fatty molecules in cells throughout the body. It contains a version of Gal A, made with Protalix’s proprietary plant cell-based system ProCellEx, with a special chemical modification called PEGylation.
This modification is designed to make Gal A more stable in the blood, providing a therapy with a long half-life — the time required for a medication’s levels to reduce by half in the blood.
Who can take Elfabrio?
The U.S. Food and Drug Administration approved Elfabrio in May 2023 for the treatment of adults with confirmed Fabry disease.
That same year, the therapy also was approved in the European Union and in the U.K. for the same indication.
Who should not use Elfabrio?
There are no contraindications for, or specific restrictions against, Elfabrio’s use.
However, the prescribing information for Elfabrio has a boxed warning noting that some patients may experience severe allergic reactions to the medication, including a life-threatening reaction called anaphylaxis. Appropriate medical support measures, such as cardiopulmonary resuscitation equipment, should be readily available during Elfabrio’s administration. If a severe hypersensitivity reaction occurs, the therapy should be immediately discontinued and appropriate treatment should be started.
How is Elfabrio administered in Fabry disease?
Elfabrio is given via an intravenous infusion, which is administered every other week in an infusion center or at a doctor’s office. It’s available as single-dose vials containing 20 mg of pegunigalsidase alfa in 10 mL of a clear and colorless solution.
The medication is given at a dose of 1 mg per kilogram of body weight. Before each infusion, patients may be treated with anti-inflammatory medications like antihistamines or corticosteroids to reduce the risk of allergic reactions, especially if they received pretreatment with these medications in prior ERT administrations.
For the first 4-6 infusions, the infusion rate, or the speed at which the medication is administered, is determined based on the patient’s body weight. Slower infusion rates are used for patients who’ve never been on an enzyme replacement therapy before starting Elfabrio. Those who have previously been on an ERT can start at slightly higher rates.
If a patient experiences mild or moderate infusion reactions, the infusion may be paused for 15 to 30 minutes, or the infusion rate may be slowed by 25% to 50%. If the reaction persists despite pausing or slowing treatment, the infusion should be stopped and the patient should be monitored. A new infusion may be given a week or two later at a slower infusion rate and with appropriate pretreatment to manage infusion reactions.
If the first four to six infusions are tolerated well, subsequent infusions can be shortened. It’s recommended that the total infusion time be reduced by 30 minutes every third infusion, down to a minimum recommended infusion time of 1.5 hours.
If a scheduled dose is missed, an infusion should be given as soon as possible and then every two weeks thereafter. The dose shouldn’t be increased to compensate for missed infusions.
In patients who reach an infusion duration that’s well tolerated, home administration under the supervision of a healthcare provider may be considered. If that is recommended by a healthcare provider, patients must continue to receive the infusion in a duration that was well tolerated in the clinic. Reductions in infusion durations should only be done in a healthcare facility.
Elfabrio in clinical trials
The approvals of Elfabrio were based on data from a comprehensive clinical program involving more than 140 patients. This program included three Phase 3 clinical trials: BRIDGE, BALANCE, and BRIGHT.
BRIDGE
The BRIDGE trial (NCT03018730) was an open-label study that enrolled 22 adults with Fabry who were receiving agalsidase alfa for at least two years. Agalsidase alfa is an older enzyme replacement therapy developed by Takeda that is sold as Replagal in several countries, but never gained approval in the U.S.
Participants were screened for three months while on agalsidase alfa, then switched to Elfabrio, which was given via infusion every two weeks for about one year.
Switching therapies was generally well tolerated, meeting the trial’s main goal. Five patients experienced treatment-related side effects and two discontinued Elfabrio due to immune reactions against the therapy.
Elfabrio also slowed the decline in kidney function, as assessed with a measure called estimated glomerular filtration rate, or eGFR. This measure is calculated in mL per minute per 1.73 meters squared. More specifically, average eGFR scores worsened by 5.9 per year with agalsidase alfa, compared with an annual 1.19 reduction on Elfabrio — a difference of 4.7.
All but two of the patients had stable or improved eGFR over the course of the study, and improvements were similar in male and female participants. After a year of treatment, nine (45%) of the patients had improved such that they were classified as having less severe kidney disease. There also were three patients who were classified as having more severe kidney disease. The rest experienced no change.
Data also suggested Elfabrio reduced levels of the Fabry disease biomarker globotriaosylsphingosine (lyso-Gb3), by 31.5% on average. The decrease in lyso-Gb3 was more pronounced among men, who generally had higher levels of this biomarker going into the trial.
BALANCE
The BALANCE study (NCT02795676) was designed to compare the impact of Elfabrio versus Fabrazyme (agalsidase beta) — an approved ERT sold by Sanofi Genzyme — on kidney function.
A total of 77 adults with Fabry and impaired kidney function were enrolled. All were receiving treatment with Fabrazyme for at least a year, and were then randomly assigned to continue on Fabrazyme or switch to Elfabrio for about two years. Both therapies were given via infusion every two weeks at a dose of 1 mg/kg.
Top-line results showed both therapies had comparable effects on kidney function. After two years, the median eGFR worsened by 2.39 for patients on Elfabrio and 3.2 with Fabrazyme. Based on historical patient data, this decline would be expected to be at 7 if patients had received no treatment.
Each of the therapies also stabilized the levels of lyso-Gb3 in the overall study population. Increases in lyso-Gb3 exceeding 20% and 10 nanomolar from the trial’s start were seen in 26 patients — 18 on Elfabrio and 8 on Fabrazyme — all of whom were men with signs of kidney dysfunction.
Safety data also were comparable. Side effects related to treatment were reported in about 40% of patients on either therapy. Two patients discontinued Elfabrio during the study, including one who experienced an allergic reaction. The results showed patients on Elfabrio were less likely to experience infusion-related reactions.
BRIGHT trial
The BRIGHT trial (NCT03180840) was launched to evaluate the safety and efficacy of a less frequent dosing regimen of Elfabrio. It enrolled 30 adults with Fabry who had been receiving a commercially available ERT (Fabrazyme or agalsidase alfa) every other week for at least three years.
All patients in BRIGHT were switched to Elfabrio, which was given at a dose of 2 mg/kg every four weeks. Of the 29 patients who completed one year of treatment, almost all maintained the less-frequent dosing regimen, except for one who was switched to 1 mg/kg given every two weeks due to clinical worsening.
Top-line data showed relatively stable kidney function with monthly Elfabrio infusions. The average rate of eGFR worsening was 1.27 at one year. Levels of the disease biomarker lyso-Gb3 also remained generally stable, increasing from 19.36 nanomolar (nM) at the study’s start to 22.23 nM after one year, and to 22.98 nM after about two years. There were no substantial Fabry-related clinical events reported during the study.
Patients’ self-rated quality of life — measured on a scale from 0 to 100 using the Quality of Life EQ-5D-5L questionnaire — was generally good at the start of the trial, with a mean score of 78.3. After one year in the study, average quality of life scores remained high, even showing a slight increase, at 82.1. Based on responses to the short-form Brief Pain Inventory (BPI) questionnaire, three-quarters of patients (75%) had no pain worsening after a year in BRIGHT.
Common side effects of Elfabrio
The most common side effects related to Elfabrio treatment in clinical trials include:
- sinus inflammation (sinusitis) and the common cold (nasopharyngitis)
- headache
- diarrhea
- fatigue
- nausea
- pain in the back and/or limbs.
Allergic reactions
Elfabrio’s prescribing information carries a boxed warning noting that hypersensitivity reactions — meaning exaggerated or inappropriate immune responses — have been reported in patients given the therapy. The risk of such allergic reactions may be increased in patients who are positive for antibodies targeting the delivered Gal A enzyme.
Prior to Elfabrio’s administration, patients may receive pretreatment with antihistamines, anti-fever medications, or corticosteroids to prevent or reduce the severity of these allergic reactions. Appropriate medical support measures should be available at all times during an infusion.
If mild or moderate reactions occur, the infusion may be paused or slowed. Should a severe allergic reaction occur, the medication should immediately be discontinued, and appropriate support should be given.
Infusion-associated reactions
Infusion-associated reactions have been reported in about one-third of patients who received Elfabrio in clinical trials. These are defined as any adverse event that occurs during or shortly after Elfabrio is administered, and may include nausea, vomiting, abdominal pain, burning sensations, itchy skin, and shortness of breath.
Infusion-associated reactions also are more common in patients with antibodies against the delivered Gal A enzyme, and can be managed with pretreatment. Patients with compromised cardiac function should be closely monitored for these reactions due to a higher risk of complications.
Kidney damage
A case of membranoproliferative glomerulonephritis — a potentially severe form of kidney damage — was reported in a patient given Elfabrio in a clinical trial. Markers of kidney function should be regularly monitored with Elfabrio; if a patient shows signs of kidney damage, the therapy should be discontinued until a full kidney evaluation can be performed.
Use in pregnancy and breastfeeding
There are no available clinical data on the use of Elfabrio during pregnancy although, as an enzyme replacement therapy, Elfabrio is not expected to cause problems for the mother or her unborn child. Studies in animal models generally have shown no adverse effects on embryonic development when Elfabrio was given at doses more than three times higher than what’s used with Fabry disease.
A registry study is collecting data for people with Fabry disease who become pregnant while on Elfabrio. Healthcare providers can report data to the registry by phone at 1-888-661-9260 or online.
It’s also not known if Elfabrio can be found in human milk, or has any effects on milk production or on the nursing infant. It’s recommended that patients and their doctors carefully weigh the benefits of breastfeeding against the potential risks of Elfabrio on the developing baby in determining treatment.
Fabry Disease News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Myeloid bodies may aid in Fabry diagnosis, treatment monitoring
- Life must go on, even when Fabry disease symptoms flare up
- Fabry disease treatment ST-920 improves kidney function: Data
- Unexpected health events are part of my life with Fabry disease
- Blood biomarkers may reflect disease activity, kidney function
- Real-world study shows burden of heart, other complications in Fabry
- Study identifies eye changes in Fabry that could affect vision
- 5 tips for helping teens with Fabry disease to thrive
- Death of bone tissue a rare, painful Fabry complication: Case report
- Long-term agalsidase alfa shows benefits as Fabry disease treatment
Related articles