
Fabry disease treatment and management
Last updated Aug. 15, 2024, by Lindsey Shapiro, PhD

While no cure is yet available for Fabry disease, a rare genetic disorder, there are several treatment options that can help ease Fabry disease symptoms. Treatment can slow the progression of organ damage, improve quality of life, and prolong Fabry disease life expectancy for affected patients.
Fabry disease is caused by mutations in the GLA gene, which lead to a faulty or missing alpha-galactosidase A (alpha-Gal A) enzyme. Because alpha-Gal A is necessary for breaking down a fatty substance called globotriaosylceramide (Gb3) in cells, a lack of functional enzyme causes Gb3 to toxically accumulate in the body’s tissues. This buildup is what causes the progressive organ damage and other symptoms that characterize Fabry disease.
Approved Fabry disease treatment options work in different ways to boost alpha-Gal A activity levels and lower Gb3 accumulation, helping slow Fabry progression. Depending on a person’s disease course, other medications for managing specific Fabry symptoms may also be used.
It is generally agreed that the earliest possible start to treatment offers the best outcomes. Prompt initiation of therapies can help delay serious organ involvement and keep patients as healthy as possible for as long as possible. Additionally, Fabry disease life expectancy with treatment may increase as more patients begin targeted treatment at an earlier age.
As with all chronic conditions, building an appropriate multidisciplinary care team to monitor a patient’s disease progression, and adjust the treatment plan as needed, is crucial for managing life with Fabry disease.
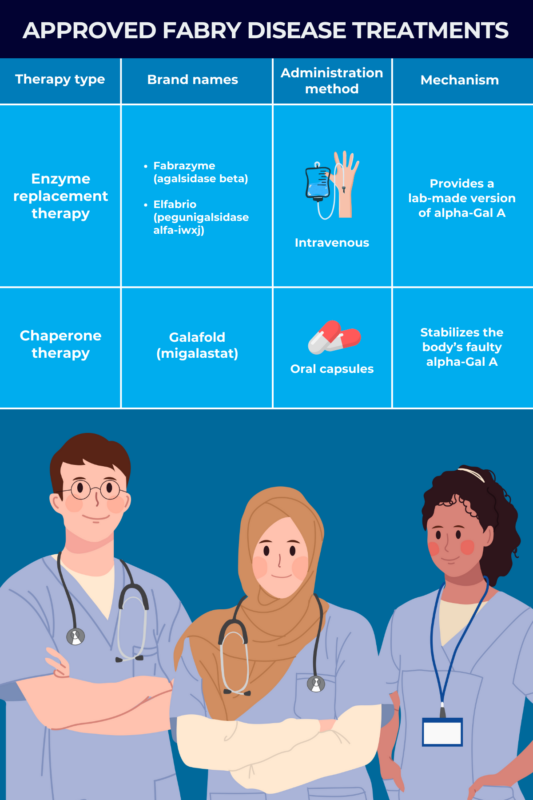
Enzyme replacement therapy
Enzyme replacement therapy, or simply ERT, is a main class of Fabry disease treatment that’s been used for more than two decades to treat Fabry patients with all types of disease-causing mutations.
It aims to provide a working, lab-made version of the alpha-Gal A enzyme. More of the enzyme in the body means that Gb3 can be broken down more effectively, which is expected to ease Fabry symptoms and slow the progression of organ damage.
ERT can be used for individuals with both of the two main disease types: classic Fabry disease, usually starting in childhood, and late-onset Fabry disease, generally manifesting after age 30. The therapy is administered via intravenous, or into-the-vein, infusions once every two weeks. Patients on ERT will require lifelong infusions to maintain adequate alpha-Gal A levels.
Two types of ERTs are approved in the U.S. for managing Fabry disease:
- Fabrazyme (agalsidase beta), from Sanofi, is approved for all patients, ages 2 and older, with confirmed Fabry disease. This enzyme is virtually identical to naturally produced alpha-Gal A.
- Elfabrio (pegunigalsidase alfa-iwxj), marketed by Chiesi Global Rare Diseases, is approved for adults with confirmed Fabry disease. It contains a special chemical modification to extend its presence in the bloodstream.
Another ERT that works similarly to Fabrazyme and Elfabrio, called agalsidase alfa, is approved in the European Union and other regions, where it is sold under the brand name Replagal. Agalsidase alfa does not have regulatory approval in the U.S., however.
The side effect profile of the ERT will depend on the specific product being used, and patients should always discuss such risks with their doctor. Some possible complications that can arise from ERT use include:
- allergic reactions
- infusion-related reactions
- headache
- fatigue
- development of antibodies against the lab-made enzyme.
Chaperone therapy
Chaperone therapy is another main class of Fabry disease treatment. It emerged more recently as an option for patients whose disease-causing mutations result in a faulty version of alpha-Gal A that is misfolded and dysfunctional.
This treatment approach aims to stabilize a person’s own faulty enzyme and make sure it folds into the proper structure. Ultimately, this allows more enzyme to be transported to the cellular compartments where it is needed to break down Gb3, thereby lowering levels of the fatty substance and preventing organ damage.
Because chaperone therapy does not provide patients with a new version of alpha-Gal A the way that enzyme replacement therapy does, it is dependent on the presence of a naturally existing enzyme. Chaperone therapy is thus not intended for patients whose GLA mutations yield no alpha-Gal A production at all.
Galafold (migalastat) is currently the only chaperone therapy approved in the U.S. for treating adults with a confirmed Fabry disease diagnosis who have amenable mutations. Marketed by Amicus Therapeutics, it is taken as an oral capsule every other day.
The most common side effects of Galafold include:
- headache
- the common cold
- urinary tract infection
- nausea
- fever.
Treatments in development
A number of experimental therapies for Fabry disease are also being tested. They are in various stages of preclinical and clinical development, and it is not known when or if these therapies will earn regulatory approval for use in Fabry patients.
Gene therapy
Gene therapies in clinical development for Fabry disease aim to provide patients with a healthy copy of the GLA gene, enabling the body’s cells to continuously produce a functional version of the alpha-Gal A enzyme. As such, gene therapy holds promise as a one-time treatment approach, although the long-term effects are still being investigated.
Among the gene therapies being developed for Fabry disease are:
- ST-920 (isaralgagene civaparvovec), a liver-targeted gene therapy from Sangamo Therapeutics
- AMT-191, a liver-targeted gene therapy from uniQure
- 4D-310, a gene therapy from 4D Molecular Therapeutics designed for enhanced heart delivery.
Gene-editing therapies, which directly modify a person’s own DNA to treat a disease, are also being investigated. However, none have yet reached clinical trials.
Substrate reduction therapy
Substrate reduction therapy, or SRT, is a treatment approach that intends to lower the production of Gb3, thereby bypassing the need for the missing or deficient alpha-Gal A enzyme.
SRTs now in development for Fabry are all designed to block an enzyme called glucosylceramide synthase, which is needed to produce Gb3 and related molecules. All are being evaluated in clinical trials, and include:
- venglustat, now owned by Sanofi
- lucerastat from Idorsia
- AL1211, being tested by Acelink Therapeutics.
Symptom management
In addition to disease-modifying therapies that directly address the underlying Fabry disease causes, treatment for Fabry disease might include interventions to manage specific symptoms as they arise.
Because it is a variable disease, Fabry disease symptom management will likely look different from person to person, and the types of treatment each patient needs might change over time as the disease evolves. Routine Fabry disease testing, including blood and urine tests, as well as hearing and heart evaluations, will help doctors know what is most suitable.
Kidney disease
As part of Fabry disease management, a person’s kidney function will be routinely monitored via urine and/or blood tests. If patients show signs of kidney disease, oral medications prescribed by clinicians might include angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARBs), which help to lower blood pressure and reduce kidney stress that causes damage.
If kidney disease progresses to the point of end-stage renal disease, or kidney failure, patients may need dialysis or a kidney transplant.
Heart disease
A range of different heart problems can occur in Fabry, requiring various types of medications and interventions. This may include, but is not limited to:
- ACEIs and ARBs for controlling blood pressure
- medications to control the heart’s rhythm and rate
- treatments to prevent blood clotting
- pacemakers, which are surgically implanted devices that help the heart beat normally.
As with kidney disease, Fabry patients will have their heart function routinely monitored by their doctors or other healthcare providers. If the disease progresses to the point where the heart can no longer effectively pump blood, a person may require a heart transplant.
Risk of stroke
Fabry patients who are at risk of having a stroke — where blood flow in the brain is blocked, depriving certain regions of oxygen — may be prescribed blood thinners. These work in different ways to prevent blood clots and can be categorized as:
- antiplatelet agents (e.g. aspirin or clopidogrel)
- anticoagulants (e.g. heparin or warfarin).
By adopting a healthier lifestyle, patients can reduce their risk factors for stroke. Managing blood pressure through appropriate medications, such as ACEIs or ARBs, and using lipid-lowering drugs to manage cholesterol levels can also contribute to stroke prevention.
Hearing problems
Fabry disease patients who experience some degree of hearing loss may require hearing aids. A surgically inserted device, called a cochlear implant, might be an option in cases of profound deafness. Infusions of steroids or vasodilators, which improve blood flow, may be used in instances of sudden hearing loss.
Vertigo, a sensation of spinning related to inner ear damage, may also occur in people living with Fabry and require the use of anti-nausea medications.
Gastrointestinal symptoms
Gastrointestinal symptoms are common and early manifestations of Fabry disease. Dietary changes, including modifying the amount, timing, and types of foods consumed, may help to ease or minimize these symptoms.
Depending on the nature of the symptoms, certain medications might also be used. These can be broadly divided into four classes:
- anti-diarrheal treatments
- medications to ease nerve-related abdominal pain
- treatments to lower inflammation in the gastrointestinal tract
- anti-nausea medications.
There are many different medications available for treating gastrointestinal issues. A person’s doctor will help select the best ones based on the individual’s symptoms and needs.
Skin problems
Angiokeratomas, or hard bumps on the skin, are a common manifestation of Fabry that occur due to the spread of dilated blood vessels in the skin. These usually are benign and not painful, and don’t specifically require treatment. However, some treatment options exist to address issues such as lesion bleeding, itching, or cosmetic issues. These include:
- laser ablation, which uses high-intensity light beams to clear the skin and minimize any associated scarring
- cryotherapy, which uses freezing temperatures to remove skin lesions
- electrosurgery, which uses heat to destroy skin lesions
- skin excision to remove damaged blood vessels.
Pain
Nerve-related pain in Fabry disease can be exacerbated by things such as heat, infection, or dehydration. As Fabry impacts a person’s ability to sweat, patients typically have difficulty regulating their temperature and may experience pain crises upon exertion or exposure to extreme temperatures.
Lifestyle changes to avoid these triggers are an important aspect of pain control in Fabry. This might include staying cool or avoiding the sun, quickly treating fever or illness, and staying hydrated. Patients also may consider shorter exercise routines and taking showers before and after each workout session.
For managing chronic pain, a person’s doctor might suggest the routine use of any of a number of oral medications. Different medicines usually act in different ways to influence the nervous system pathways involved in pain. Examples of medication options include:
- carbamazepine
- gabapentin
- phenytoin
- pregabalin
- antidepressants.
If a person arrives at the clinic having an acute pain crisis, the individual might be treated with oral or intravenous (into-the-vein) medications or nonsteroidal anti-inflammatory medicines. Such oral or intravenous drugs may include opioid treatments such as tramadol, morphine, or oxycodone. Anti-inflammatory drugs can include ibuprofen or diclofenac.
Healthcare providers will carefully choose pain medications to make sure they don’t interact with any other treatments being used by the patient, and to ensure they’re safe based on that person’s kidney function.
Building a care team
Gb3 accumulation can affect virtually all tissues in the body, so Fabry disease patients experience a wide range of symptoms that can change over time. After receiving a Fabry disease diagnosis, proper disease management will require a multidisciplinary care team who together can monitor how the disease is progressing and come up with a Fabry disease treatment plan that addresses the patient’s individual needs.
Depending on their symptoms, patients may need to regularly meet with various medical specialists, including:
- nephrologists, or kidney doctors
- cardiologists
- ophthalmologists
- neurologists
- audiologists, or specialists in hearing-related issues
- otolaryngologists, or specialists in ear, nose, and throat.
Managing a chronic condition like Fabry disease can take a toll on a person’s mental health and well-being. A psychologist or other mental healthcare professional, who can help patients talk through their feelings and develop coping strategies, might thus also be a critical member of a Fabry patient’s care team.
With all the types of care that may be necessary, the total Fabry disease treatment cost may be burdensome for some families. Patients should work closely with their care teams to come up with the best treatment plan for their particular situation.
Fabry Disease News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Common Fabry symptoms often mimic IBS in adults
- Two of my sons share what it’s like having three siblings with Fabry
- Idorsia outlines new Phase 3 program for lucerastat in Fabry disease
- Jeff’s Journey With Fabry Disease
- Eye vessel abnormalities may signal heart disease in Fabry patients
- We need more oral Fabry disease treatment options that reduce pain
- AMT-191 shows promise, but safety concerns prompt dosing pause
- Guest Voice: Believe us when we say we’re having a bad day
- Sangamo starts FDA submission seeking approval of Fabry gene therapy
- Managing my hypertension has required some trial and error




